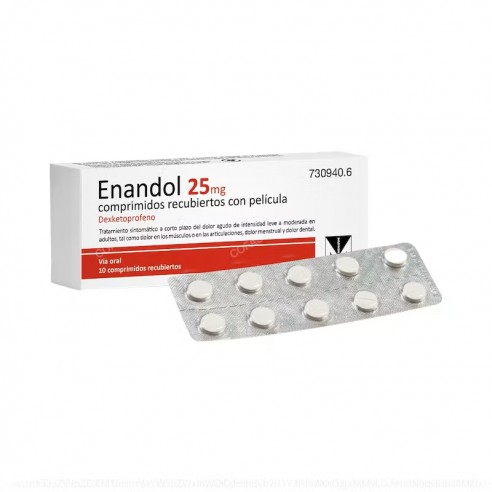
Enandol 25Mg 10 Film-coated Tablets
Indications for use of Enandol 25Mg 10 Film-coated Tablets
Enandol 25Mg 10 Film-coated Tablets is a medicine in film-coated tablet form, intended for adults over 18 years of age. It is a non-prescription medicine and is indicated for the short-term symptomatic treatment of acute pain of mild to moderate intensity, such as muscle or joint pain, menstrual pain and dental pain.
Action and mechanism of Enandol 25Mg 10 Film-coated Tablets
Enandol 25Mg 10 Film-coated Tablets contains dexketoprofen as the active substance. This is an active ingredient of the non-steroidal anti-inflammatory drugs (NSAIDs) group, which exerts its anti-inflammatory and analgesic action by inhibiting the synthesis of prostaglandins. It does this by inhibiting the enzymes cyclo-oxygenase-1 (COX-1) and cyclo-oxygenase-2 (COX-2).
Dosage
The recommendation is to take 1 tablet (25 mg dexketoprofen) every 8 hours, not exceeding 3 tablets per day (75 mg). Treatment should not exceed 4 days.
Contraindications
Do not take in any of the following cases:
- If allergic to dexketoprofen or other non-steroidal anti-inflammatory drugs, or to any of the excipients of the medicinal product.
- During the last 3 months of pregnancy or while breast-feeding.
- Read the package leaflet carefully to find out about all cases.
Pharmacist's recommendations
- We recommend that you take the medicine with food, thus reducing the risk of stomach upset.
- do not exceed 4 days of treatment.
- if the pain persists after 4 days, worsens or new symptoms appear, consult your doctor.
Adverse reactions
The most frequently observed adverse effects have been gastrointestinal.
Storage
Do not store above 30º C.
Composition
Active substance: dexketoprofen. Each tablet contains 25 mg dexketoprofen.
Excipients: Tablet core: maize starch, microcrystalline cellulose, sodium carboxymethyl starch (Type A) (potato starch), glycerol distearate. Tablet coating: dry lacquer consisting of: hypromellose, titanium dioxide and macrogol 6000, and propylene glycol (E-1520).